Cuttlefish Hunting Behavior: Figures and Tables
Figures and tables for the paper “An experimental method for evoking and characterizing dynamic color patterning of cuttlefish during prey capture” (in prep):
Figures
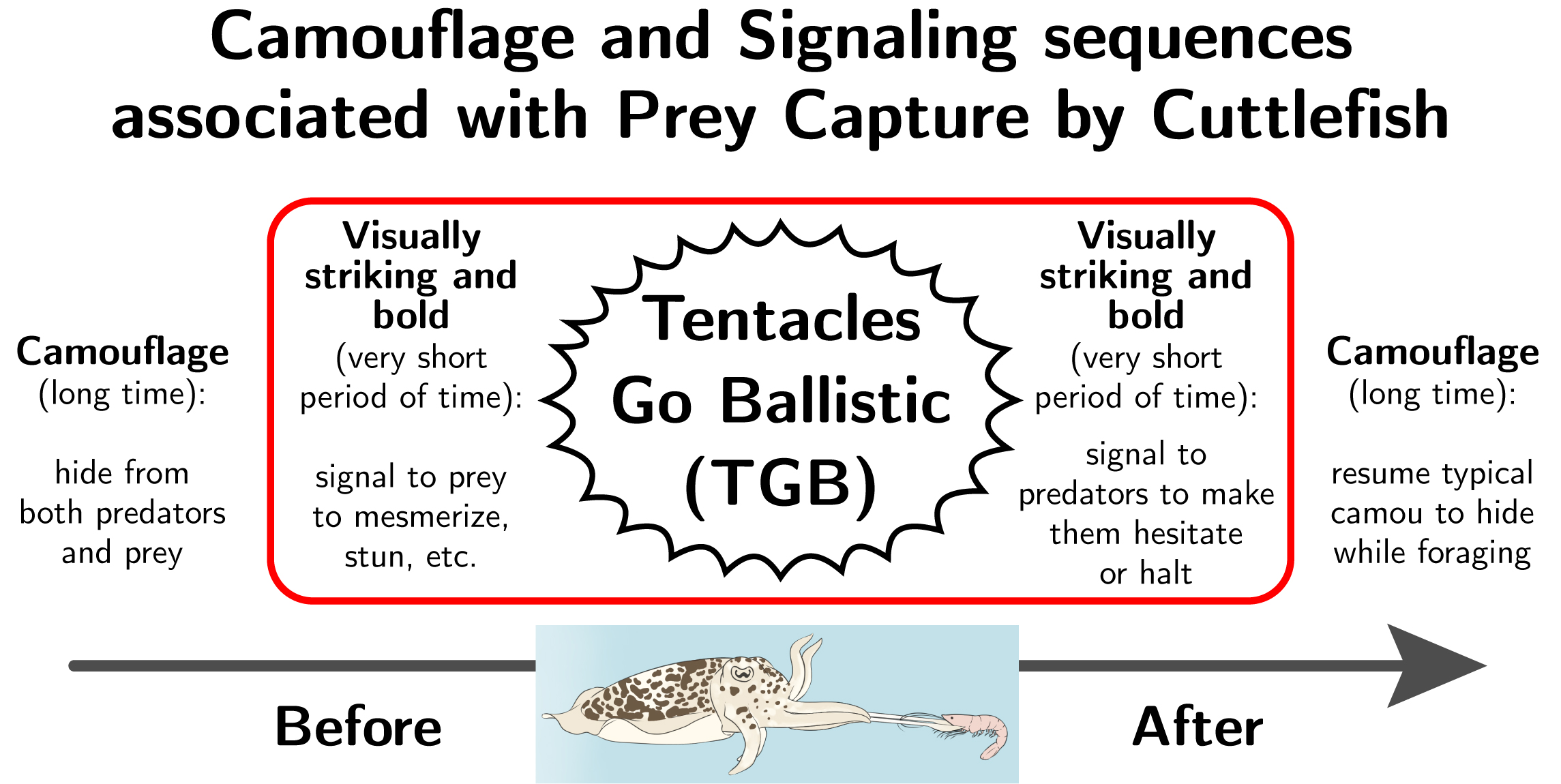
Figure 1: Camouflage and signaling sequences associated with prey capture by cuttlefish. Tentative framework for the sequence of body patterning during prey capture (see also [7]). This study focuses on the 3 seconds immediately before and after prey capture, circled in red in this diagram. Illustration: Jennifer Deutscher.
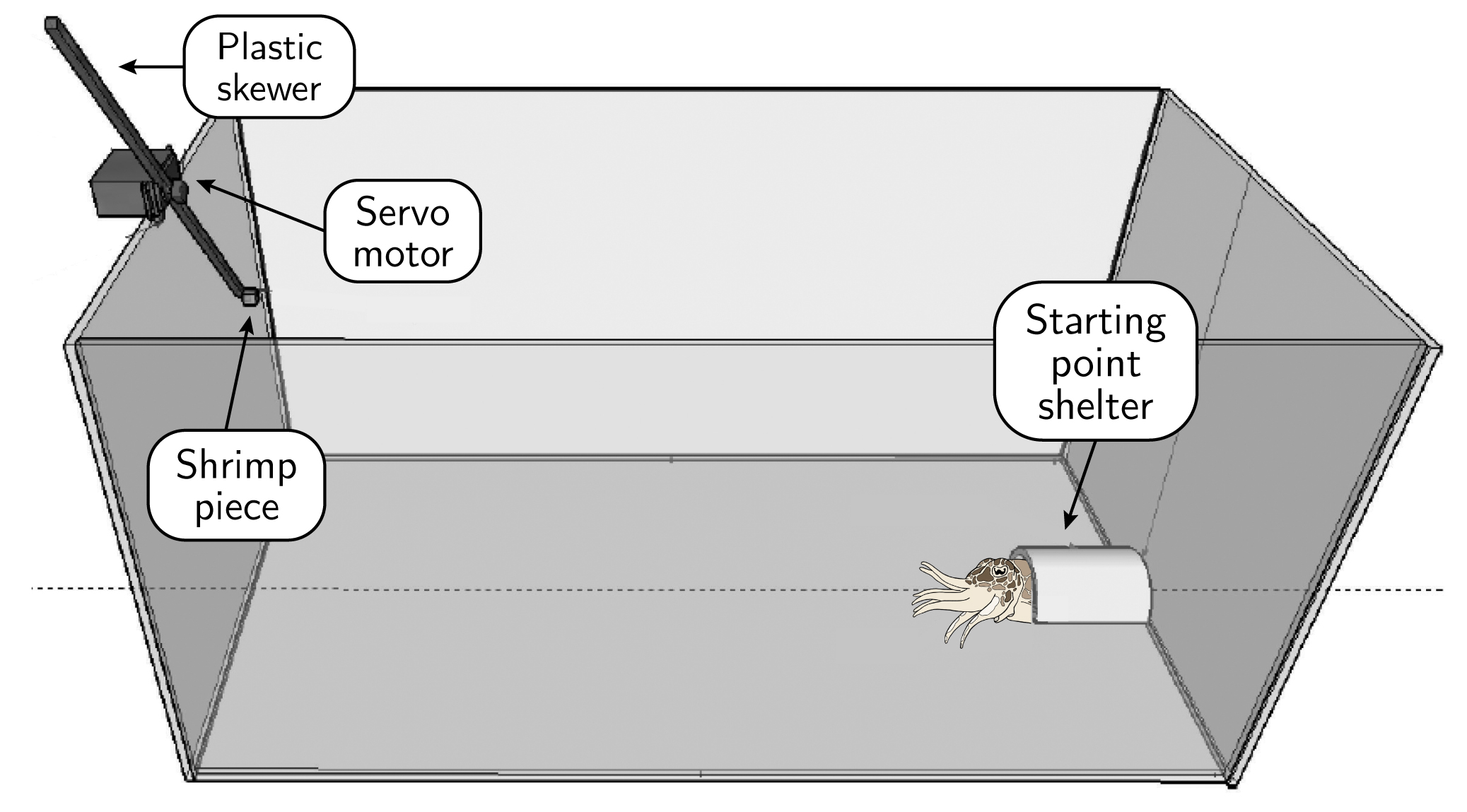
Figure 2: Experimental tank setup (not to scale). An acrylic box (43 x 43 x 81 cm, open top) with ‘robotic prey’ (shrimp piece on a plastic skewer moved by an Arduino-controlled servo motor) on the left, perched on top of the experimental tank wall, and “starting point shelter” on the right, where cuttlefish can hide during acclimation. An overhead camera (not shown) provided a top-down, monochrome recording of the setup, while an underwater camera (also not shown) recorded from another angle in color.
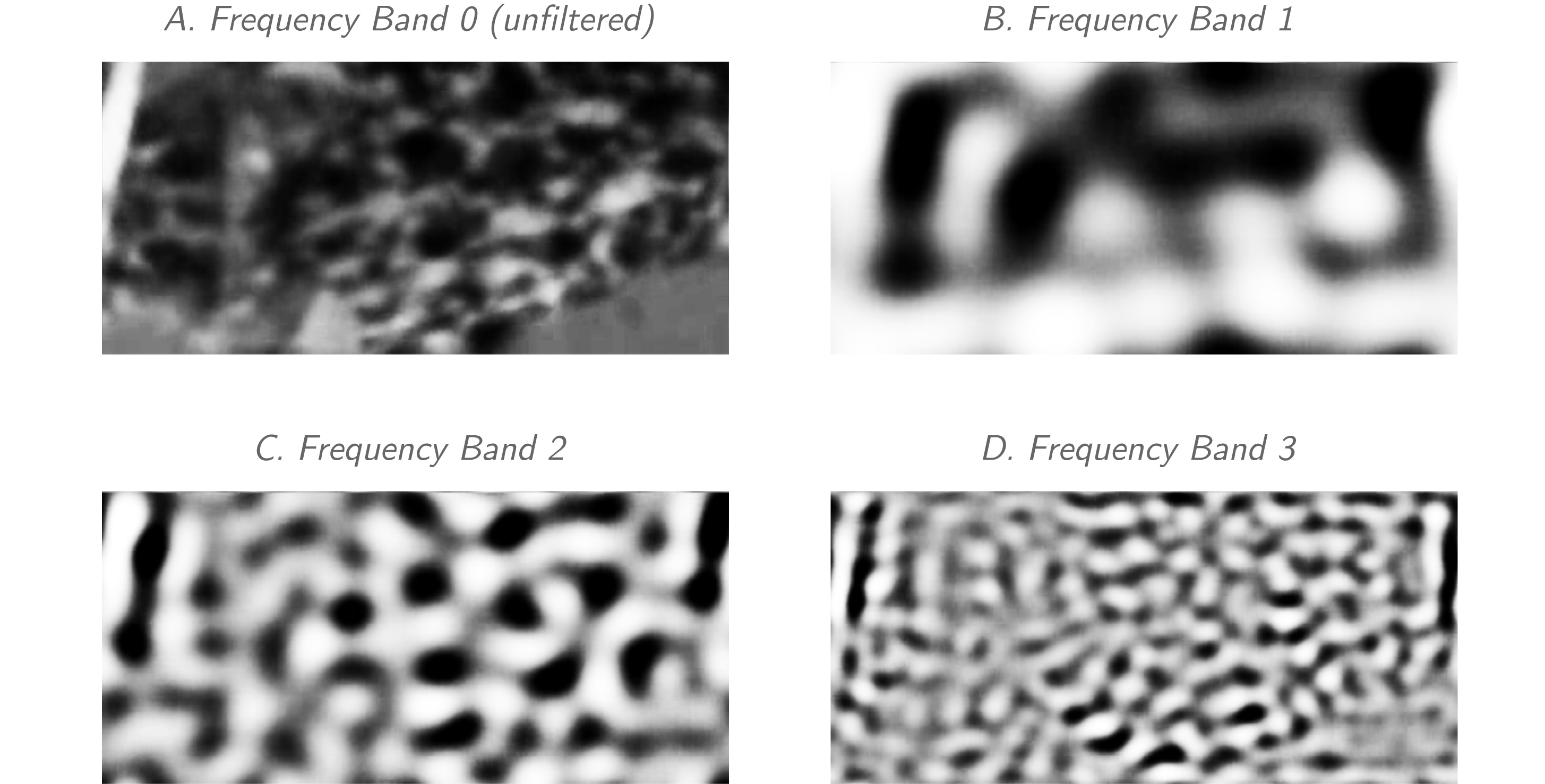
Figure 3: Example screenshot of mantle body pattern after filtering for analysis. A screenshot of the mantle body pattern (+1000ms after TGB) is shown after filtering through the 4 frequency bands used by Process Cuttle Python (modified from [21]) to analyze the TSP.
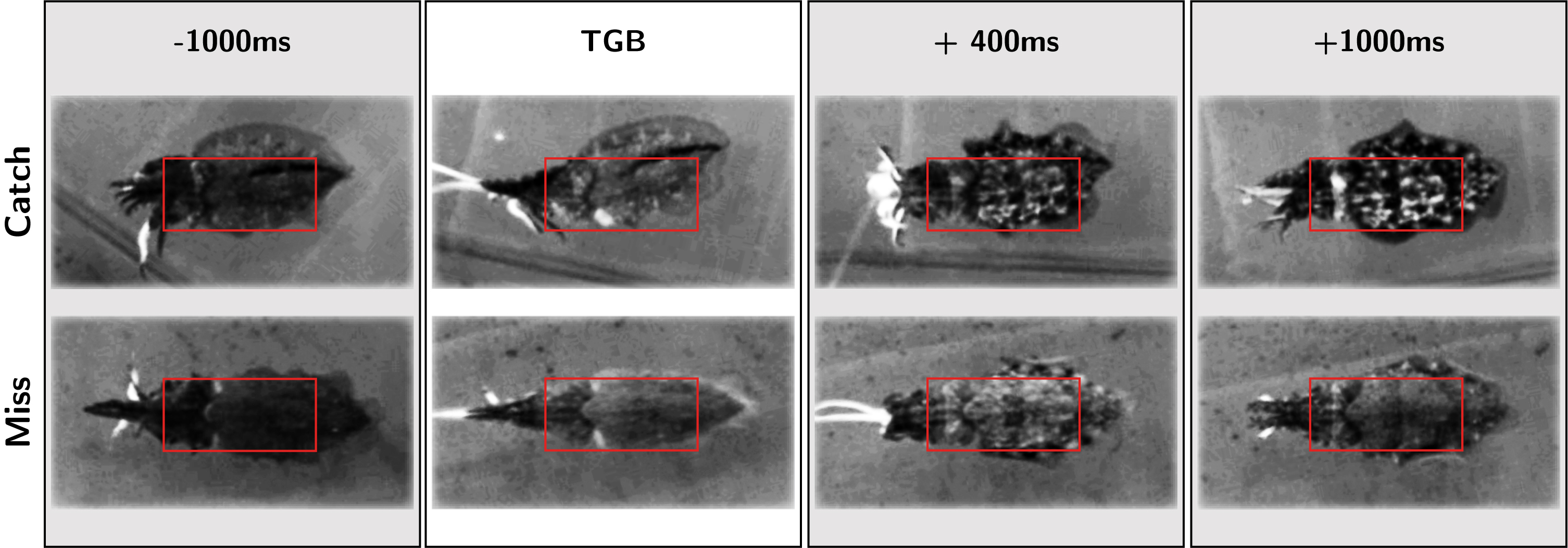
Figure 4: Ethogram of body pattern changes during hunting behavior in captive Sepia officinalis (exemplary screenshots from overhead video). The top row shows body pattern changes (TSP) during a successful attack, while the bottom row shows body pattern changes during an unsuccessful attack. All images are frames from manually cropped and aligned video clips of tentacle shots made by the same animal. Red boxes indicate ROI described in Methods section ‘Video analyses – characterizing the TSP’.
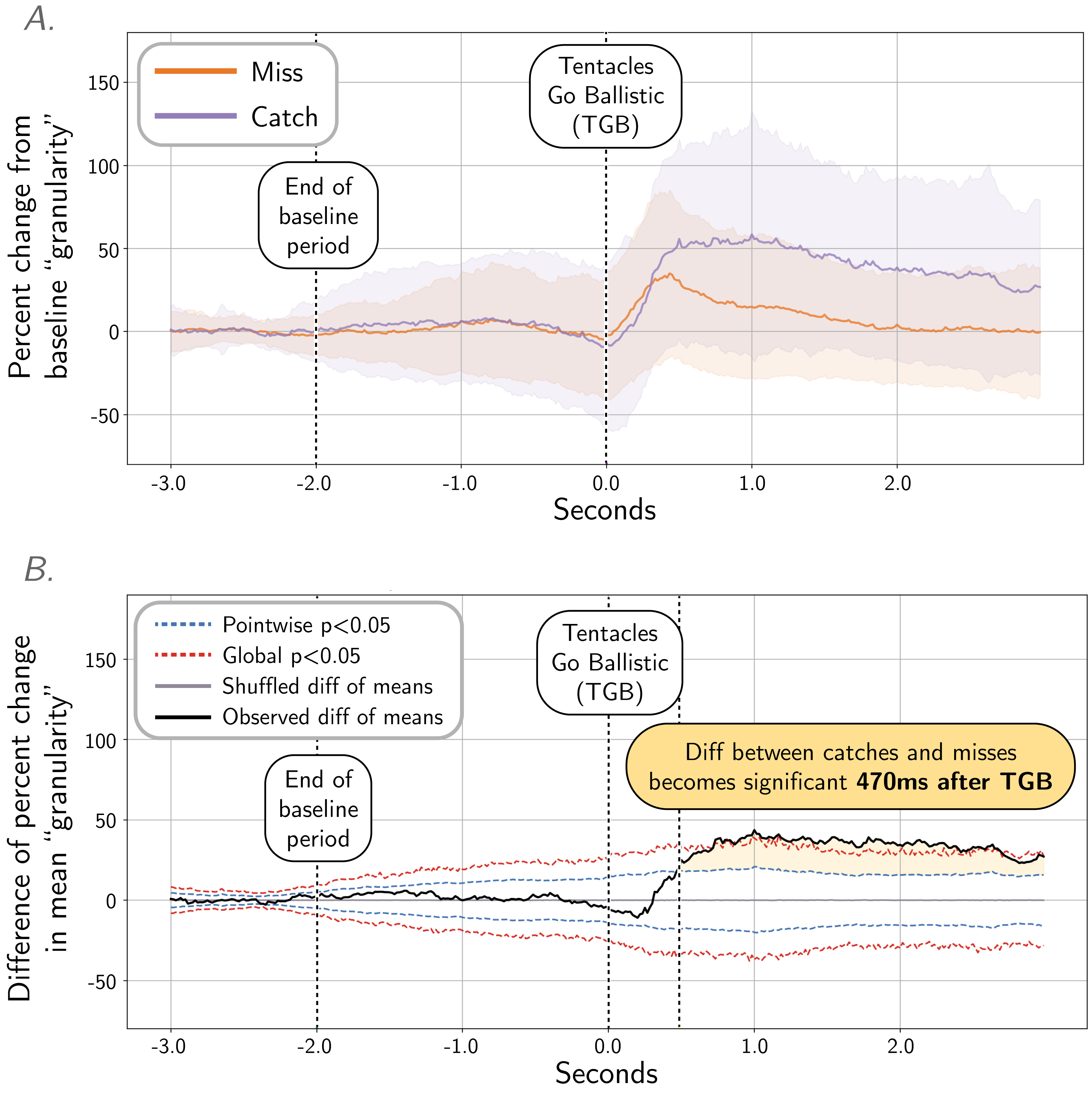
Figure 5: Quantifying the dynamics of TSPs with ‘granularity’ analysis, frequency band 2. A. Mean ‘granularity’ of body pattern during tentacle shots in frequency band 2 (measured by Process Cuttle Python, modified from [21]), from 3 seconds before TGB to 3 seconds after TGB, normalized and pooled across all subjects. B. A shuffle test (N=20,000 shuffles) of the difference of means (catch vs. miss) show significance at 470ms after TGB. See Figure S8 for granularity analysis and shuffle test plots at all spatial frequencies.
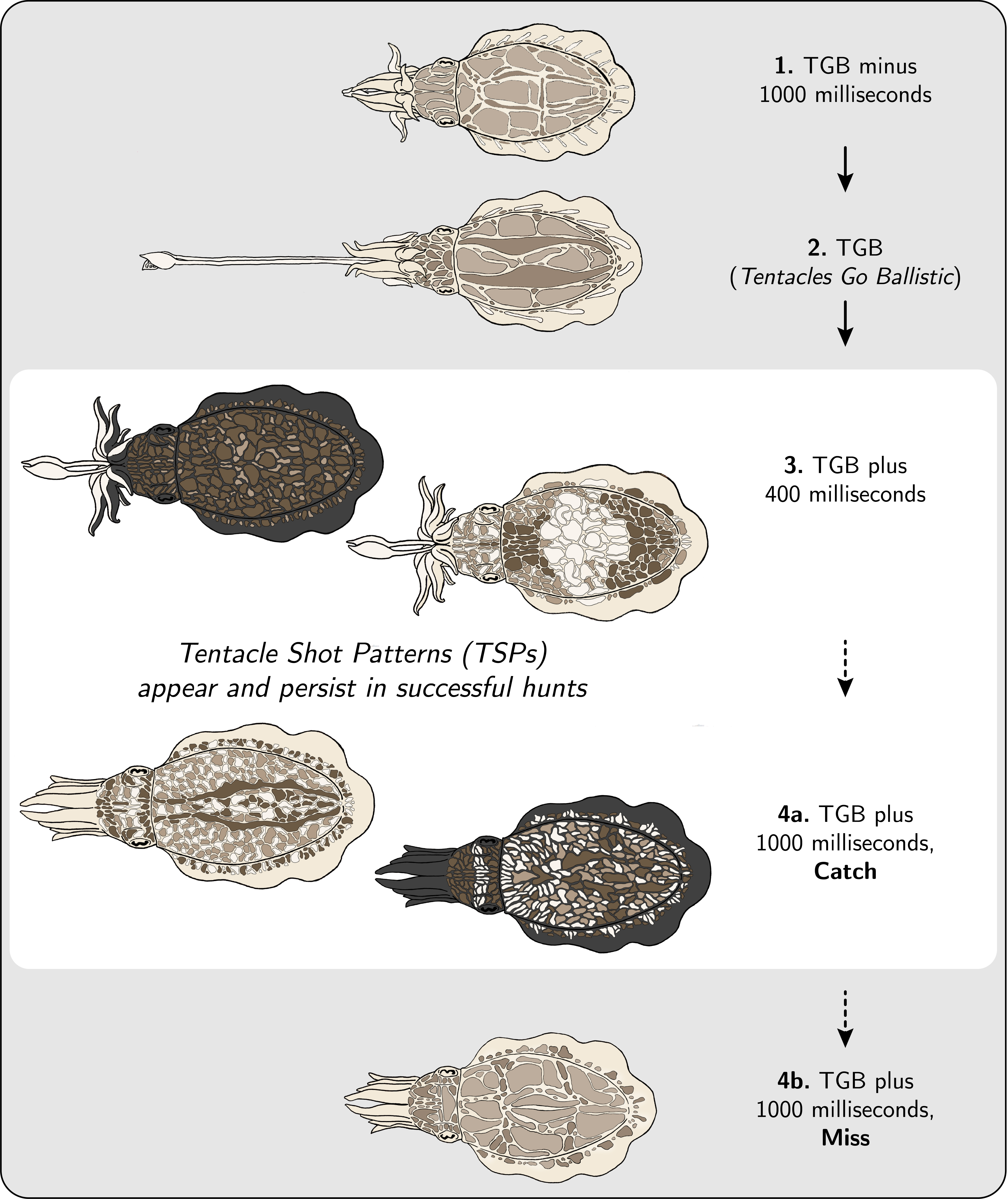
Figure 6: Schematic of body pattern changes during prey capture, shown in 4 stages. 1. TGB minus 1000 milliseconds: Animal is typically transitioning from the positioning phase to the seizure phase of the hunt (see [8]), and tentacles are just beginning to show from within the arms. 2. TGB (Tentacles Go Ballistic): This is the moment when tentacles are ballistically released towards the prey or food item. 3. TGB plus 400 milliseconds (Tentacle Shot Patterns appear): By 400 milliseconds after TGB, the ‘granularity’ of the deployed body pattern has increased significantly as compared to the ‘granularity’ of the body pattern deployed during baseline (TGB minus 3000 milliseconds to TGB minus 2000 milliseconds). 4a. TGB plus 1000 milliseconds, Catch (TSPs persist): When the hunt is successful, the ‘granularity’ of the deployed body pattern remains high. See Fig. 7 for all examples of TSPs in the dataset. 4b. TGB plus 1000 milliseconds, Miss (TSPs disappear): When the hunt is unsuccessful, the ‘granularity’ of the deployed body pattern returns to baseline. Illustration: Danbee Kim.

Figure 7: Examples of Tentacle Shot Patterns. This composite image shows screenshots of all tentacle shots by all animals at 233.5 milliseconds after TGB, the timepoint when TSPs are most strongly displayed, regardless of whether the animal caught or missed the prey.
Tables
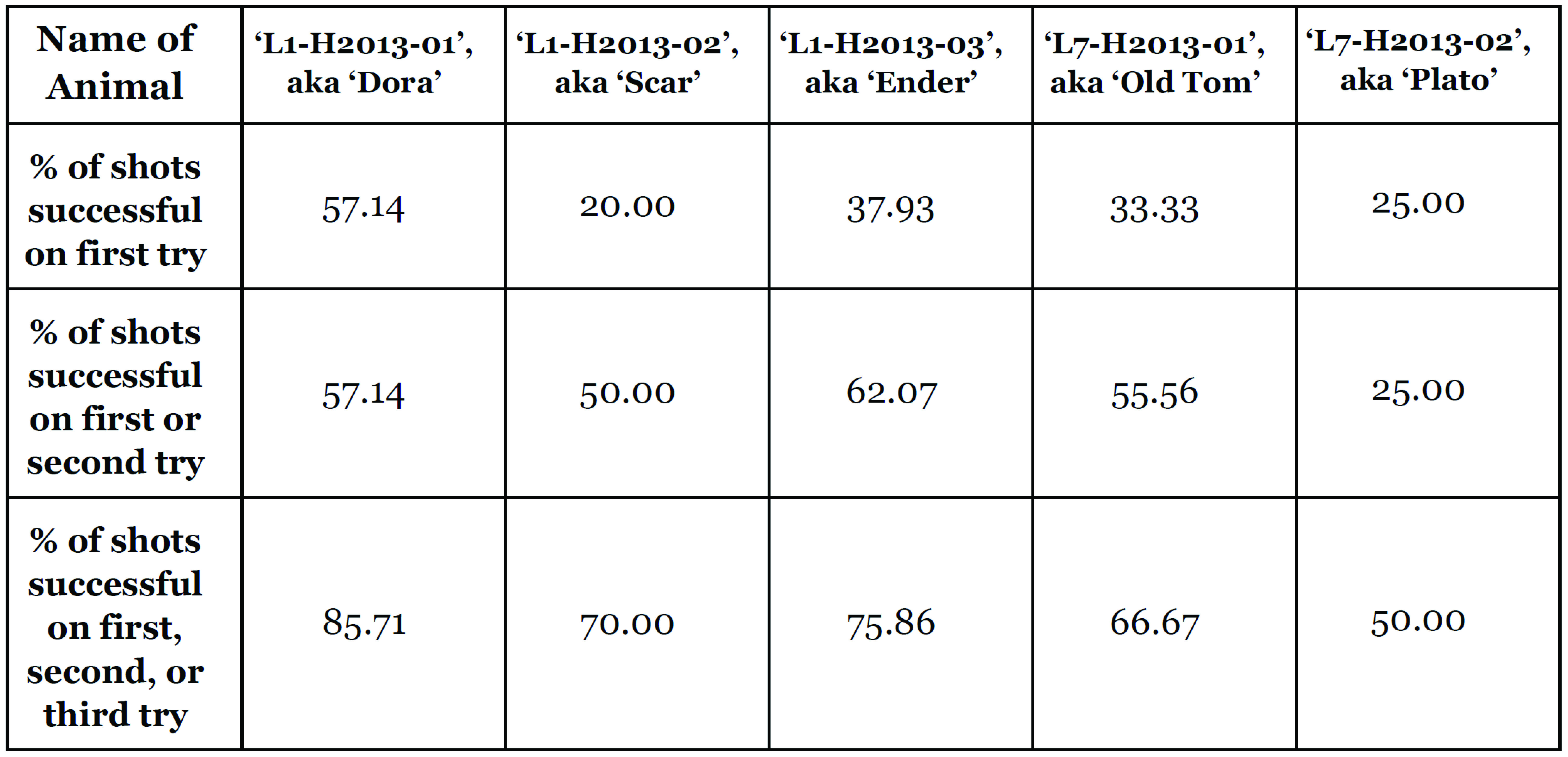
Table 1: Accuracy of seizure via tentacle shot while hunting robotic prey, for all animals throughout the entire experimental protocol.
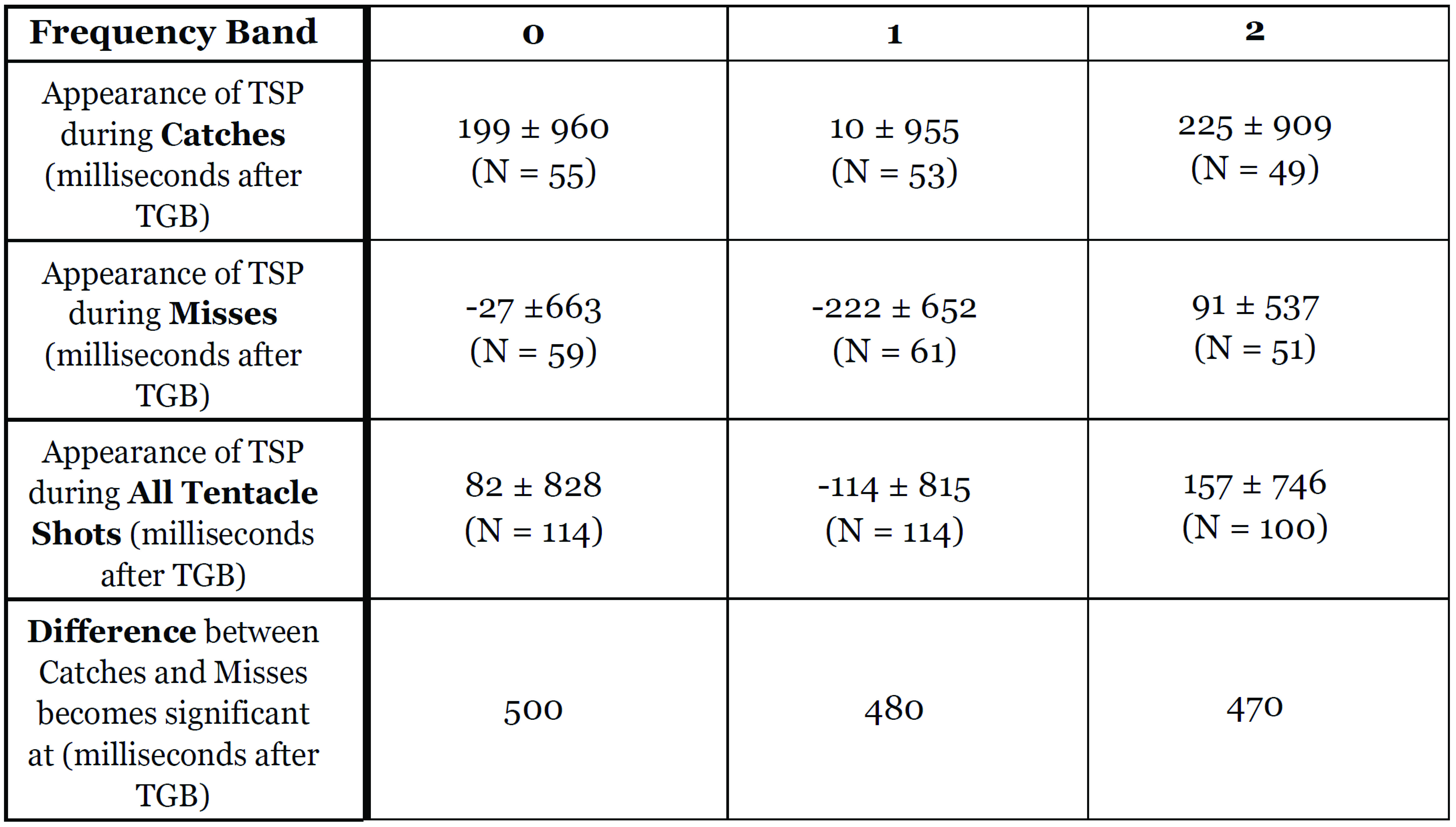
Table 2: Summary of TSP dynamics, showing mean timing of appearance of TSP, and timing of significant (p<0.05) divergence between successful and unsuccessful hunts.
References
- Adamo SA, Ehgoetz K, Sangster C, Whitehorne I (2006) Signaling to the enemy? Body pattern expression and its response to external cues during hunting in the cuttlefish Sepia officinalis (Cephalopoda). The Biol Bull 210(3):192-200.
- Holmes W (1940) The colour changes and colour patterns of Sepia officinalis L. Proceedings of the Zoological Society of London 110 (1-2): 17-35.
- How MJ, Norman MD, Finn J, Chung WS, Marshall NJ (2017) Dynamic skin patterns in cephalopods. Front Physiol 19(8):393.
- Langridge KV (2006) Symmetrical crypsis and asymmetrical signalling in the cuttlefish Sepia officinalis. Proceedings of the Royal Society B: Biological Sciences 273(1589):959-67.
- Langridge KV, Broom M, Osorio D (2007) Selective signalling by cuttlefish to predators. Current Biology 17(24):R1044-5.
- Shashar N, Rutledge P, Cronin T (1996) Polarization vision in cuttlefish in a concealed communication channel?. Journal of Experimental Biology 199(9):2077-84.
- Hanlon RT, Messenger JB (2018) Cephalopod behaviour. Cambridge University Press.
- Messenger JB (1968) The visual attack of the cuttlefish, Sepia officinalis. Animal Behaviour 16(2-3):342-57.
- Sereni E (1930) The chromatophores of the cephalopods. The Biol Bull 59(3):247-68.
- Thompson DW (1910) Aristotle: Historia animalium. The works of Aristotle translated into English, book IV. The Classical Review.
- Cole PD, Adamo SA. Cuttlefish (Sepia officinalis: Cephalopoda) hunting behavior and associative learning (2005) Anim cogn 8(1):27-30.
- Duval P, Chichery MP, Chichery R (1984) Prey capture by the cuttlefish (Sepia officinalis L): an experimental study of two strategies. Behavioural processes 9(1):13-21.
- Feord RC, Sumner ME, Pusdekar S, Kalra L, Gonzalez-Bellido PT, Wardill TJ (2020) Cuttlefish use stereopsis to strike at prey. Sci Adv 6(2):eaay6036.
- Zylinski S, Osorio D, Shohet AJ (2009) Cuttlefish camouflage: context-dependent body pattern use during motion. Proceedings of the Royal Society B: Biological Sciences 276(1675):3963-9.
- Hanlon RT, Messenger JB (1988) Adaptive coloration in young cuttlefish (Sepia officinalis L.): the morphology and development of body patterns and their relation to behaviour. Philosophical Transactions of the Royal Society of London. B, Biological Sciences 320(1200):437-87.
- Carreno Castilla A, Hernandez-Urcera J, Gouraguine A, Guerra Á, Cabanellas-Reboredo M (2020) Predation behaviour of the European squid Loligo vulgaris. J Ethol 38:311-22.
- Gordon D, Pugh P, Cooke GM (2019) Social Media and citizen science provide valuable data for behavioural ecology research: Are cuttlefish using pursuit-deterrent signals during hunting?. bioRxiv: 760926.
- Lopes G, Nogueira J, Dimitriadis G, Menendez JA, Paton JJ, Kampff AR (2017) A robust role for motor cortex. bioRxiv: 058917
- Panetta D, Solomon M, Buresch K, Hanlon RT (2017) Small-scale rearing of cuttlefish (Sepia officinalis) for research purposes. Mar Freshw Behav Physiol 50(2):115-24.
- Lopes G, Bonacchi N, Frazão J, Neto JP, Atallah BV, Soares S, Moreira L, Matias S, Itskov PM, Correia PA, Medina RE (2015) Bonsai: an event-based framework for processing and controlling data streams. Front Neuroinform 9:7.
- Barbosa A, Mäthger LM, Buresch KC, Kelly J, Chubb C, Chiao CC, Hanlon RT (2008) Cuttlefish camouflage: the effects of substrate contrast and size in evoking uniform, mottle or disruptive body patterns. Vision Res 48(10):1242-53.
- Packard A, Hochberg FG (1977) Skin patterning in Octopus and other genera. In Symp. Zool. Soc. Lond 38: 191-231.
- Graving JM, Chae D, Naik H, Li L, Koger B, Costelloe BR, Couzin ID (2019) DeepPoseKit, a software toolkit for fast and robust animal pose estimation using deep learning. Elife 8:e47994.
- Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, Bethge M (2018) DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci 21(9):1281-9.
- Nath T, Mathis A, Chen AC, Patel A, Bethge M, Mathis MW (2019) Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat Protoc 14(7):2152-76.
- Laan A, Gutnick T, Kuba MJ, Laurent G (2014) Behavioral analysis of cuttlefish traveling waves and its implications for neural control. Current Biology 24(15):1737-42.
- Reiter S, Hülsdunk P, Woo T, Lauterbach MA, Eberle JS, Akay LA, Longo A, Meier-Credo J, Kretschmer F, Langer JD, Kaschube M (2018) Elucidating the control and development of skin patterning in cuttlefish. Nature 562(7727):361-6.
Leave a Comment